 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

Catalyst In a PEM fuel cell, the oxygen reduction reaction at the cathode is the kinetically slow process which is plays a key role for the performance of the entire fuel cell. Consequently, it is very important to develop and improve active catalysts for the oxygen reduction reaction whereby activity, cost and stability of the catalyst are the most relevant criteria.
There are two main approaches: Two issues with regard to the first approach are the development of alloyed, low-Pt content catalysts that do not display compromised performance, and the improvement of Pt utilization by optimizing the catalyst layers. |
| ||||
|
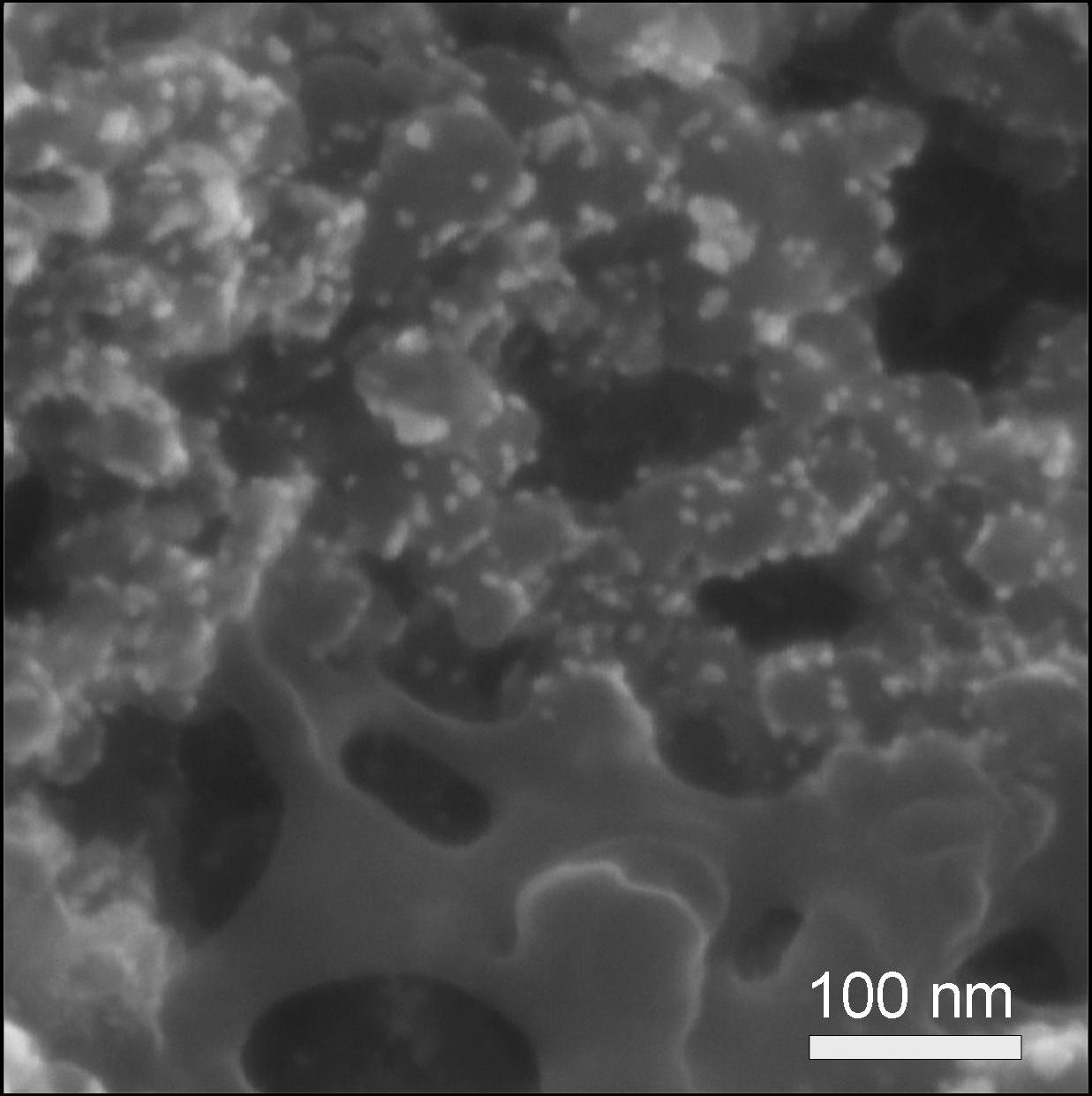
Non-noble metal catalysts might be considered the most feasible way to permanently resolve the cost issue for fuel cell commercialization, especially considering the development of Pt prices in the recent years. Even though activity and stability challenges are still far from being suitable for practical PEM fuel cell applications, the improvement of this type of catalysts might be essential for a sustainable application of PEM fuel cells. The figure shows a scanning electron micrograph of Pt-based catalyst particles (bright little particles). They have a diameter of a few nanometer, which is typical for this Pt-based catalysts. It is also possible to image catalyst particles by transmission electron microscopy - even with better resolution. Image published in: A. Pfrang, Fuel cell testing – Degradation of fuel cells and its impact on fuel cell applications, GIT Laboratory Journal Europe 2009, 13(3-4), 42-44
| |||||