 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

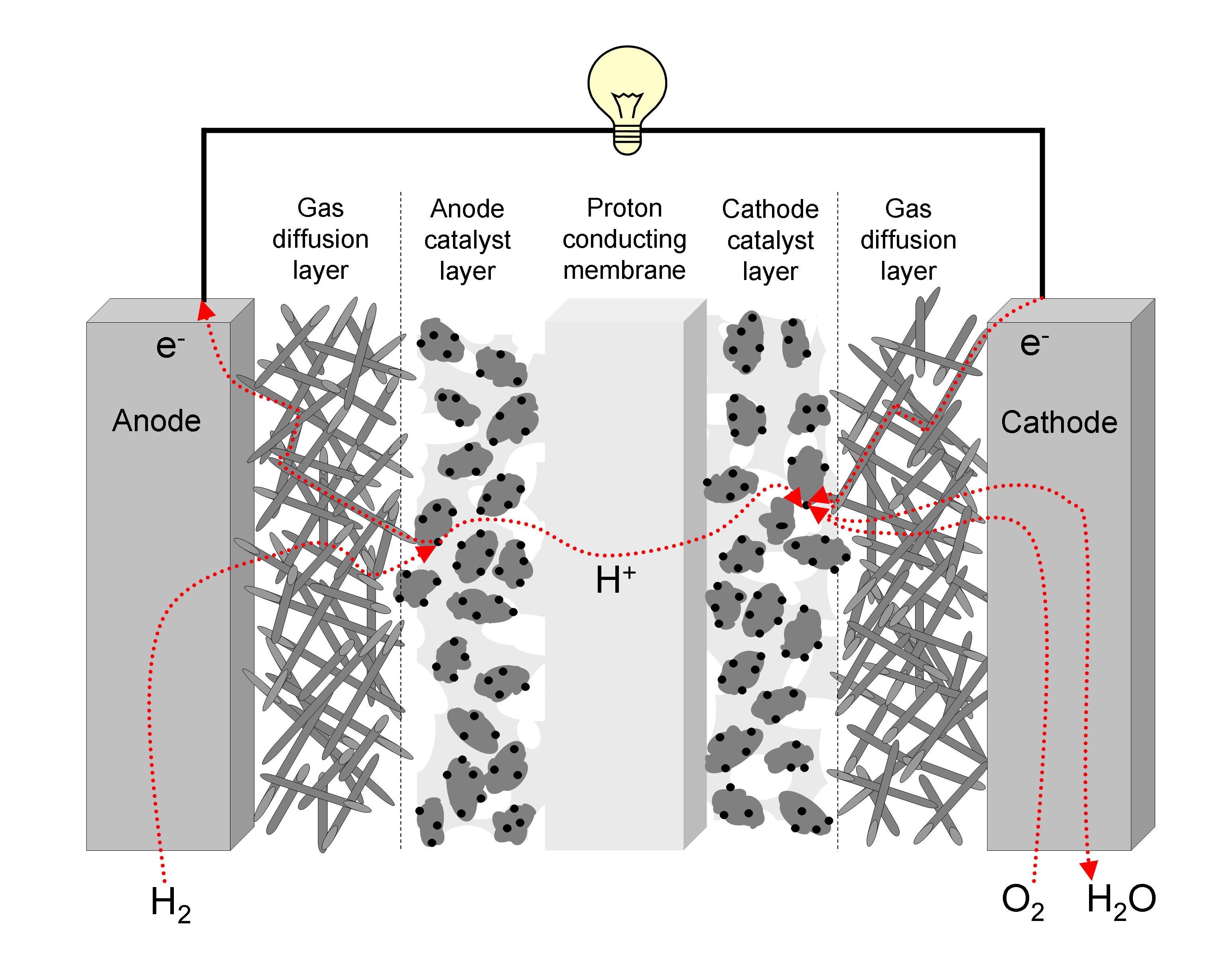
Polymer Electrolyte Membrane (PEM) Fuel Cells Fuel cells in general are expected to play an important role in the future energy supply and in the long-term could partly substitute current power generation technologies in all end use sectors. Fuel cells can combine high conversion efficiency with sustainability, especially when hydrogen produced from renewable energy sources is used as fuel. But even when using conventional fuels such as natural gas for fuel production, they have, in the medium and long-term, a high potential for a large reductions of CO2 emissions and energy savings. Fuel cells convert chemical energy in electrical energy and heat by consuming typically hydrogen and oxygen and producing water. This is achieved by reducing hydrogen at the anode (left hand side) and oxidising oxygen at the cathode (right hand side). |
| ||||
|
The protons produced on the anode side move through the proton-conducting (but not electron-conducting) membrane to the cathode side. Electrons will flow via the electrical load outside the fuel cell to the cathode side. Overall, this results in the production of water as 'waste': 2 H2 + O2 → 2 H2O For some types of fuel cells, hydrogen can be replaced by other hydrogen-containing fuels as e.g. methanol. Instead of using pure oxygen also normal air can be used for operation of the fuel cell. Despite the simple basic principle of fuel cells, the operation under real conditions is far from completely understood in detail and some challenging technological issues have to be solved.Polymer electrolyte membrane (PEM) fuel cells are promising candidates for mobile applications e.g. in cars due to the relatively short start-up times and the high flexibility of the delivered power. On the other hand, PEM fuel cells require pure hydrogen as fuel and noble metals as catalyst which results in relatively high cost. The lifetime requirements for mobile applications can be achieved for PEM fuel cells at high loadings of noble metals and under laboratory conditions. At lower noble metal loadings – which is preferable in terms of cost – and under real-world conditions – which includes load cycling, start-stop cycles, varying humidification, fuel starvation, temperatures above 90 °C and mechanical vibration – fuel cell degradation is still an important issue. Typical degradations mechanisms include chemical degradation or mechanical failure of the membrane, loss of electrochemically active surface area (ECSA) of the catalyst and chemical and mechanical degradation of the gas diffusion layer.
Image published in: A. Pfrang, Fuel cell testing – Degradation of fuel cells and its impact on fuel cell applications, GIT Laboratory Journal Europe 2009, 13(3-4), 42-44
| |||||